Abstract
Background: Glofitamab is a T-cell-engaging bispecific monoclonal antibody with a novel 2:1 (CD20:CD3) configuration that confers bivalency for CD20 on B cells and monovalency for CD3 on T cells. Pivotal results from the global Phase I/II NP30179 study (NCT03075696) of glofitamab monotherapy indicate high response rates and manageable safety in patients with heavily pretreated, highly refractory diffuse large B-cell lymphoma (DLBCL); obinutuzumab pretreatment and Cycle (C) 1 step-up dosing effectively mitigated cytokine release syndrome (CRS; Dickinson et al. ASCO 2022). Here, we report results from a Phase I study (NCT04657302) of fixed-duration glofitamab monotherapy in Chinese patients with DLBCL after ≥2 prior lines of therapy.
Methods: Eligible patients had DLBCL (DLBCL not otherwise specified [NOS]; high-grade B-cell lymphoma [HGBCL]; primary mediastinal B-cell lymphoma [PMBCL]; transformed follicular lymphoma [trFL]) and had received ≥2 prior regimens including ≥1 anti-CD20 antibody and ≥1 anthracycline. Patients received intravenous (IV) obinutuzumab pretreatment (1000mg) on C1 Day (D) 1, followed by step-up doses of IV glofitamab on C1D8 (2.5mg) and C1D15 (10mg), and the target dose (30mg) on D1 of C2-12 (21-day cycles). This study was designed to evaluate the pharmacokinetics (PK), safety, tolerability, and efficacy of fixed-duration glofitamab monotherapy. The primary efficacy endpoint was complete response (CR) rate as assessed by Independent Review Committee (IRC) using Lugano 2014 criteria (Cheson et al. J Clin Oncol 2014). Secondary efficacy endpoints included overall response rate (ORR), duration of complete response (DOCR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Responses were assessed by PET-CT. CRS was assessed by ASTCT criteria (Lee et al. Biol Blood Marrow Transplant 2019). Other adverse events (AEs) were assessed by CTCAE v5.0.
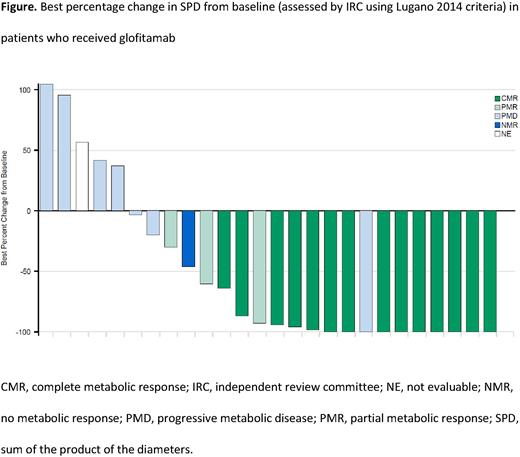
Results: As of May 30, 2022, 30 patients were enrolled, of whom 27 had been treated with ≥1 dose of glofitamab (median age: 58 years [range: 20-82]; Ann Arbor stage III-IV disease: 90%; Eastern Cooperative Oncology Group Performance Status 0/1: 50%/50%; bulky disease: 37%; DLBCL-NOS: 67%; HGBCL: 10%; PMBCL: 17%; trFL: 7%). Median number of prior therapies was 2 (range: 2-6), 67% of patients were primary refractory, 90% were refractory to their most recent regimen, and 20% had previously received chimeric antigen receptor T-cell (CAR-T) therapy (13% of these patients were refractory to CAR-T). The PK profile of glofitamab in Chinese patients appeared to be bi-phasic, and increased dosing (2.5mg‒30mg) showed dose-proportionality, which was also observed in the global NP30179 trial population. No post-dose anti-glofitamab anti-drug antibodies were identified. At data cut-off, the median follow-up was 10 months. Anti-tumor activity was observed in most patients (Figure). ORR and CR rates by IRC were 63% and 52%, respectively. Median time to CR was 43 days. Median DOR and DOCR were not reached. The majority of ORRs (13/17; 76%) and CRs (11/14; 79%) were ongoing at data cut-off. IRC-assessed median PFS was 8 months (95% CI: 3‒not evaluable [NE]). Median OS was 11 months (95% CI: 9‒NE). Serious AEs were reported in 43% of patients and there were no fatal AEs. AEs leading to treatment discontinuation were reported in two patients (7%). CRS events were the most common AEs (63%; grade [gr] 1: 57%; gr 2: 3%; gr 3: 3%), and all events were resolved at data cut-off. CRS events occurred predominantly during C1; no gr ≥2 CRS events occurred after C2. Neurologic AEs were reported in 27% of patients (gr 1: 17%; gr 2: 7%; gr 3: 3%). No glofitamab-related neurologic AEs potentially consistent with immune effector cell-associated neurotoxicity syndrome were reported.
Conclusions: The PK profile of glofitamab in Chinese patients was similar to that reported in the global NP30179 pivotal cohort. Consistent with the NP30179 study, high response rates and durable responses were achieved in patients receiving glofitamab, and the safety profile of glofitamab in this Chinese population was manageable. These data suggest clinically meaningful outcomes with fixed-duration glofitamab monotherapy in heavily pretreated and highly refractory patients with R/R DLBCL from China.
Disclosures
Wang:Roche (China) Holding Ltd.: Current Employment; Roche: Current equity holder in private company. Wu:Roche (China) Holding Ltd.: Current Employment; Roche: Current equity holder in publicly-traded company. Li:Roche (China) Holding Ltd.: Current Employment. Dai:Roche R&D Center (China) Ltd.: Current Employment. Humphrey:Roche: Current equity holder in private company, Current holder of stock options in a privately-held company; Roche Products Ltd: Current Employment.
OffLabel Disclosure:
Glofitamab is a full-length, humanized immunoglobulin G1 bispecific monoclonal antibody with a 2:1 (CD20:CD3) configuration that facilitates bivalent binding to CD20 on B cells, and monovalent binding to CD3 on T cells. Glofitamab redirects T cells to eliminate normal and malignant B cells. Glofitamab is an investigational agent. Obinutuzumab (Gazyva) is a CD20-directed cytolytic antibody indicated: in combination with chlorambucil, for the treatment of pts with previously untreated CLL; in combination with bendamustine followed by obinutuzumab monotherapy, for the treatment of pts with FL who relapsed after, or are refractory to, a rituximab-containing regimen; in combination with chemo followed by obinutuzumab monotherapy in pts achieving at least a PR, for the treatment of adult pts with previously untreated stage II bulky, III or IV FL. Obinutuzumab (Gazyva) is a CD20-directed cytolytic antibody indicated: in combination with chlorambucil, for the treatment of pts with previously untreated CLL; in combination with bendamustine followed by obinutuzumab monotherapy, for the treatment of pts with FL who relapsed after, or are refractory to, a rituximab-containing regimen; in combination with chemo followed by obinutuzumab monotherapy in pts achieving at least a PR, for the treatment of adult pts with previously untreated stage II bulky, III or IV FL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal